Successful application of dietary ketogenic metabolic therapy in patients with glioblastoma: a clinical study
生酮饮食代谢疗法在胶质母细胞瘤患者中的成功应用:一项临床研究
Andreas K iry t to poul os 1, Athanasios E. Evan geli ou 2*, Irene Katsanika?, loannis Bou kov in as 4, Nikolaos Foroglou5, Basilios Zountsas°, Angeliki Cheva', Vaios Niko lo poul os 8, Thomas Za rambo uk as 8, Tomas Duraj?, Thomas N. Seyfried? and Martha Spilioti1
Andreas K iry t to poul os 1, Athanasios E. Evan geli ou 2*, Irene Katsanika?, loannis Bou kov in as 4, Nikolaos Foroglou5, Basilios Zountsas°, Angeliki Cheva', Vaios Niko lo poul os 8, Thomas Za rambo uk as 8, Tomas Duraj?, Thomas N. Seyfried? and Martha Spilioti1
1 Department of Neurology, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2 Division of Child Neurology, St Luke's Hospital, Thessaloniki, Greece, 3 Department of Diet and Nutrition, Papa georgi ou General Hospital, Thessaloniki, Greece, 4 Bioclinic Thessaloniki Medical Oncology Unit, Thessaloniki, Greece, 5 Department of Neurosurgery, Aristotle University of Thessaloniki, Thessaloniki, Greece, 6 Department of Neurosurgery, St Luke's Hospital, Thessaloniki, Greece, 7 Department of Pathology, Faculty of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece, 9 Department of Biology, Boston College, Chestnut Hill, MA, United States
1 塞萨洛尼基亚里士多德大学神经内科,希腊塞萨洛尼基,2 圣卢克医院儿童神经科,希腊塞萨洛尼基,3 帕帕乔治乌总医院饮食营养科,希腊塞萨洛尼基,4 塞萨洛尼基生物诊所肿瘤内科,希腊塞萨洛尼基,5 塞萨洛尼基亚里士多德大学神经外科,希腊塞萨洛尼基,6 圣卢克医院神经外科,希腊塞萨洛尼基,7 塞萨洛尼基亚里士多德大学医学院病理科,希腊塞萨洛尼基,9 波士顿学院生物系,美国马萨诸塞州栗树山
Introduction: Glioblastoma multiforme (GBM) ranks as one of the most aggressive primary malignant tumor affecting the brain. The persistent challenge of treatment failure and high relapse rates in GBM highlights the need for new treatment approaches. Recent research has pivoted toward exploring alternative therapeutic methods, such as the ketogenic diet, for GBM.
引言:多形性胶质母细胞瘤 (GBM) 是最具侵袭性的原发性恶性脑肿瘤之一。GBM治疗失败和高复发率的持续挑战凸显了对新治疗方法的迫切需求。近期研究开始转向探索替代疗法,例如生酮饮食在GBM中的应用。
Methods: A total of 18 patients with GBM, 8 women and 10 men, aged between 34 and 75 years participated in a prospective study, examining the impact of ketogenic diet on tumor progression. The pool of patients originated from our hospital during the period from January 2016 until July 2021 and were followed until January 2024. As an assessment criterion, we set an optimistic target for adherence to the ketogenic diet beyond 6 months. We considered the therapeutic combination successful if the survival reached at least 3 years.
方法:共有18名胶质母细胞瘤(GBM)患者(8名女性和10名男性,年龄在34至75岁之间)参与了一项前瞻性研究,旨在检验生酮饮食对肿瘤进展的影响。这些患者于2016年1月至2021年7月期间来自我院,并随访至2024年1月。我们将坚持生酮饮食超过6个月设定为乐观的评估标准。若患者生存期达到至少3年,则视为治疗组合成功。
Results: Among the 18 patients participating in the study, 6 adhered to the ketogenic diet for more than 6 months. Of these patients, one patient passed away 43 months after diagnosis, achieving a survival of 3 years; another passed away at 36 months, narrowly missing the 3-year survival mark; and one is still alive at 33 months post-diagnosis but has yet to reach the 3-year milestone and is, therefore, not included in the final survival rate calculation. The remaining 3 are also still alive, completing 84,43 and 44 months of life, respectively. Consequently, the survival rate among these patients is 4 out of 6, or $66.7%$ . Of the 12 patients who did not adhere to the diet, only one reached 36 months of survival, while the rest have died in an average time of $15.7\pm6.7$ months, with a 3-year survival rate of $8.3%$ . Comparing the survival rates of the two groups, we see that the difference is $58.3%$ ( $66.7%$ versus $8.3%$ ) and is statistically significant with $p<0.05$ (0.0114) and $X^{2}=6.409\$ .
结果:在参与研究的18名患者中,有6人坚持生酮饮食超过6个月。其中1例患者在确诊43个月后去世,实现3年生存期;另1例于36个月时离世,以微小差距未达3年生存标记;还有1例确诊后存活33个月,因尚未达到3年里程碑而未计入最终生存率统计。其余3例目前仍存活,分别完成84、43和44个月生存期。因此,该组患者生存率为4/6(66.7%)。未坚持饮食的12名患者中,仅1例达到36个月生存期,其余患者平均在(15.7±6.7)个月内死亡,3年生存率为8.3%。两组生存率比较显示差异达58.3%(66.7% vs 8.3%),具有统计学显著性(p<0.05)(0.0114),X²=6.409\$。
Discussion: The outcomes observed in these patients offer promising insights into the potential benefits of the ketogenic diet on the progression of glioblastoma multiforme when compared to those who did not follow the diet consistently.
讨论:与未坚持生酮饮食的患者相比,这些患者观察到的结果为生酮饮食对多形性胶质母细胞瘤进展的潜在益处提供了有希望的见解。
KEYWORDS ketogenic, glioblastoma, diet, multiforme, metabolic, brain, tumor
关键词 生酮饮食、胶质母细胞瘤、饮食、多形性、代谢、脑部、肿瘤
1 Introduction
1 引言
Glioblastoma (GBM) is the most common primary malignant tumor of the brain and central nervous system. It accounts for $14.5%$ of all central nervous system tumors and $48.6%$ of malignant central nervous system tumors (1). Despite surgical excision (total or subtotal), followed by adjuvant radiotherapy and chemotherapy with temozolomide, patients with newly diagnosed GBM have a median overall survival of 12–18 months, with $<10%$ surviving beyond 5 years (2, 3). Additionally, GBM is an invasive tumor and usually recurs within 32–36 weeks of initial diagnosis, despite maximal treatment.
胶质母细胞瘤 (GBM) 是大脑和中枢神经系统最常见的原发性恶性肿瘤。它占所有中枢神经系统肿瘤的 $14.5%$ 和恶性中枢神经系统肿瘤的 $48.6%$ (1)。即使经过手术切除 (全切或次全切) 并辅以放疗和替莫唑胺化疗,新诊断的 GBM 患者中位总生存期仅为 12-18 个月,$<10%$ 的患者能存活超过 5 年 (2, 3)。此外,GBM 具有侵袭性,即使在接受最大程度治疗后,通常也会在初次诊断后 32-36 周内复发。
The almost universal relapse and poor long-term prognosis highlight the need for new treatment approaches. Thus, in recent years, the research community has turned to the discovery of alternative therapeutic strategies for GBM, such as dietary ketogenic metabolic therapy (KMT). Dietary KMT is defined as a synergistic precision nutrition approach, incorporating biomarker-driven ketogenic diets, fasting and fasting-mimicking diets, as well as other lifestyle interventions (4, 5). Monitoring of adherence should be performed by quantitative, unbiased biomarkers, such as the glucose-ketone index (GKI) (6). In particular, recent advancements in our understanding of cancer metabolism have led to renewed interest in Warburg’s theory of carcinogen es is (7–9). According to this theory, malignant cells are characterized by distinct structural and functional mitochondrial abnormalities, leading to compensatory metabolic dependencies. The main metabolic phenotype of GBM is a high glycolytic rate with lactic acid fermentation due to loss of efficiency in the respiratory cycle, despite ample mutational heterogeneity and secondary metabolic reprogramming (10). Unlike normal brain cells, that have evolved to metabolize ketone bodies for energy when glucose availability is low, GBM cells depend on glycolysis for growth and are unable to efficiently metabolize ketones due to impaired mitochondrial function (11–13). This metabolic deficiency isolates cancer cells from normal cells from a metabolic perspective, regardless of their somatic mutation landscape.
普遍存在的复发和不良长期预后凸显了新治疗方法的必要性。因此,近年来研究界开始探索胶质母细胞瘤(GBM)的替代治疗策略,例如生酮代谢疗法(KMT)。膳食KMT被定义为一种协同精准营养方法,整合了生物标志物驱动的生酮饮食、禁食与模拟禁食饮食以及其他生活方式干预措施(4, 5)。治疗依从性应通过葡萄糖-酮体指数(GKI)等定量、无偏见的生物标志物进行监测(6)。特别是,近期癌症代谢研究进展重新引发了人们对Warburg致癌理论的关注(7-9)。该理论认为,恶性细胞的典型特征是线粒体存在独特的结构和功能异常,从而导致代偿性代谢依赖。尽管存在显著的突变异质性和继发性代谢重编程,GBM的主要代谢表型仍是因呼吸循环效率低下导致的高糖酵解速率伴乳酸发酵(10)。与能适应低葡萄糖环境而代谢酮体供能的正常脑细胞不同,GBM细胞依赖糖酵解生长,且由于线粒体功能受损无法有效代谢酮体(11-13)。这种代谢缺陷从代谢角度将癌细胞与正常细胞区分开来,不受体细胞突变背景影响。
Based on the above observations, interest is growing in designing metabolism-based treatments for cancer in general and primary brain tumors in particular. Specifically, GBM cells lack metabolic versatility due to mitochondrial abnormalities and are largely dependent on glucose and glutamine for energy and biosynthesis (7, 8). Moreover, Warburg discovered that the excessive amount of glucose consumed by tumor tissues is fermented to lactate despite the presence of oxygen, rather than oxidized via mitochondrial respiration (6). However, it is still unknown to what degree other pure ly oxidative fuels, such as lactate, fatty acids, or ketone bodies, could contribute to cell survival and/or proliferation under relative glycolytic depletion in vivo. It is hypothesized that cancer cells cannot compensate for the simultaneous inhibition of glycolysis and glut amino lysis via ketone body metabolism due to acquired metabolic inflexibility (14). This hypothesis is strengthened by the presence of triglyceride-rich cytoplasmic lipid droplets in many malignant cancers. Recent research work has provided strong evidence that the presence of cytoplasmic lipid droplets and the aerobic fermentation commonly seen in most malignant cancers can serve together as biomarkers for oxidative phosphor yl ation inefficiency (15).
基于上述观察,人们对于设计基于代谢的癌症治疗方案,尤其是针对原发性脑肿瘤的兴趣日益增长。具体而言,由于线粒体异常,GBM细胞缺乏代谢灵活性,主要依赖葡萄糖和谷氨酰胺获取能量并进行生物合成 (7, 8)。此外,Warburg发现,尽管存在氧气,肿瘤组织消耗的过量葡萄糖仍会发酵为乳酸,而非通过线粒体呼吸氧化 (6)。然而,在体内相对糖酵解耗竭的情况下,其他纯氧化燃料(如乳酸、脂肪酸或酮体)能在多大程度上促进细胞存活和/或增殖仍不清楚。据推测,由于获得性代谢不灵活性,癌细胞无法通过酮体代谢来补偿糖酵解和谷氨酰胺分解的同时抑制 (14)。许多恶性癌症中存在富含甘油三酯的胞质脂滴,进一步支持了这一假说。最近的研究工作提供了强有力的证据,表明胞质脂滴的存在以及在大多数恶性癌症中常见的有氧发酵,可以共同作为氧化磷酸化效率低下的生物标志物 (15)。
Thus, with the current failure of che mo radiotherapy to improve long-term outcomes in GBM, as well as the proposed inability of GBM cells to efficiently oxidize ketone bodies when glycolysis is sufficiently limited, dietary KMT has been tested in the clinic to weaken the tumor and protect healthy cells during cytotoxic treatments (16, 17). Glucose and ketone metabolism in normal and cancer cells is illustrated in Figures 1A,B, 2A,B. While there is adequate experimental evidence supporting the beneficial effects of KMT on brain tumors, clinical testing is still ongoing (13, 18, 19). Most of the translational research to date involved case series and pilot clinical trials (20–24). In experimental models, KMT reduces glycolytic flux in cancer cells and increases ketolysis in non-tumoral cells, while also enhancing tumorreactive immune responses (25). Therefore, biomarker-driven ketogenic diets could be a complementary therapeutic intervention for patients with GBM, aiming to slow tumor growth, potentiate standard cytotoxic therapies, and extend longterm survival.
因此,鉴于当前化疗放疗未能改善胶质母细胞瘤(GBM)的长期预后,以及提出的当糖酵解被充分限制时GBM细胞无法有效氧化酮体的观点,生酮代谢疗法(KMT)已在临床中进行测试,旨在细胞毒性治疗期间削弱肿瘤并保护健康细胞 (16, 17)。图1A,B和2A,B展示了正常细胞与癌细胞中的葡萄糖及酮体代谢情况。虽然现有充分实验证据支持生酮代谢疗法对脑肿瘤的积极作用,但相关临床试验仍在进行中 (13, 18, 19)。目前大多数转化研究涉及病例系列和初步临床试验 (20–24)。在实验模型中,生酮代谢疗法可降低癌细胞的糖酵解通量,同时增强非肿瘤细胞的酮体分解能力,并能提升肿瘤反应性免疫应答 (25)。因此,基于生物标志物的生酮饮食可能成为GBM患者的补充治疗手段,以期延缓肿瘤生长、增强标准细胞毒性疗法效果并延长长期生存期。
Based on Warburg’s theory and expanding upon the above mentioned experimental and clinical data, we offered ketogenic diet therapy to a cohort of 18 GBM patients. GBM was chosen as a target due to well-characterized metabolic dependency upon glycolysis and poor prognosis despite maximal standard therapies (3, 26, 27). Our decision to offer ketogenic diet therapy to our patients was strengthened by the fact that no significant side effects have been reported in previous safety and feasibility studies (28, 29).
基于Warburg的理论并结合上述实验与临床数据,我们对18名GBM患者实施了生酮饮食疗法。选择GBM作为研究对象,是因为该肿瘤具有明确的糖酵解代谢依赖性,且即使采用标准疗法预后仍较差 (3, 26, 27)。此前安全性和可行性研究中未报告显著副作用 (28, 29),这一事实增强了我们对患者实施生酮饮食疗法的信心。
2 Materials and methods
2 材料与方法
2.1 Study design
2.1 研究设计
A total of 18 patients with GBM, 8 women and 10 men, between 34 and 75 years of age (median age 57.5 years) participated in our prospective study evaluating the effects of ketogenic diet therapy on tumor progression. The pool of patients originated from our hospital and from a collaborating oncologist during the period from January 2016 until July 2021 and were followed until January 2024. The study was conducted in accordance with the1975 declaration of Helsinki and received prior approval from the ethics committee of our university (PN 1232/16). A double-blind design was planned, but due to the rapid progression of the disease, a survival of 3 years or more was considered as a success factor (30, 31). All subjects were referred to our hospital’s outpatient clinic for a general neurological assessment, including neurological status and MRI evaluation. A detailed laboratory evaluation was also performed, including the following: complete blood count, biochemical tests (electrolytes, blood glucose, trans a minas es, cholesterol, triglycerides, thyroid hormones), electrocardiogram, and electroencephalogram (EEG).
共有18名GBM患者参与了我们的前瞻性研究,评估生酮饮食疗法对肿瘤进展的影响,其中女性8名,男性10名,年龄介于34至75岁之间(中位年龄57.5岁)。这些患者来自我院及合作肿瘤科医生2016年1月至2021年7月期间的接诊病例,随访持续至2024年1月。本研究遵循1975年《赫尔辛基宣言》,并事先获得我校伦理委员会批准(编号PN 1232/16)。原计划采用双盲设计,但因疾病快速进展,将3年及以上生存期视为成功指标[30,31]。所有受试者均转诊至我院门诊进行神经学综合评估,包括神经状态检查和MRI评估。同时进行详细实验室检测,项目包括:全血细胞计数、生化检验(电解质、血糖、转氨酶、胆固醇、甘油三酯、甲状腺激素)、心电图及脑电图(EEG)。
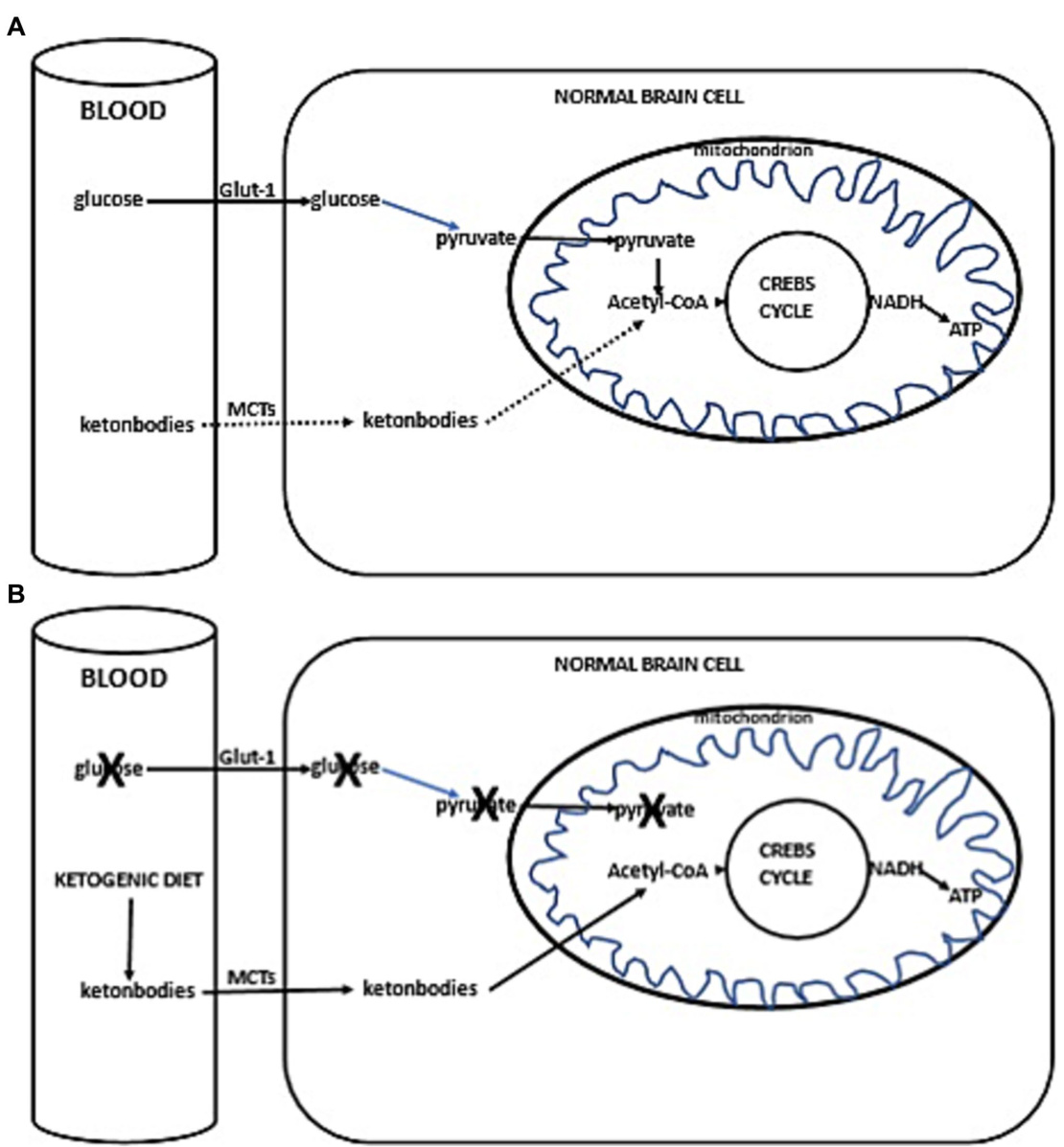
FIGURE 1 (A) Simplified scheme of glucose and ketone metabolism in a normal brain cell. Under anaerobic conditions, normal cells perform glycolysis in the cytoplasm, generating minimal but rapid energy. In aerobic conditions, normal cells perform the slower but more efficient oxidative phosphor yl ation in mitochondria for energy production. In a fed state, cellular energy is derived from glucose metabolism (illustrated with a solid line), undergoing glycolysis in the cytoplasm to form pyruvate, which then enters the mitochondrion. Inside the mitochondria, pyruvate is converted into acetyl-CoA, initiating the citric acid cycle (Krebs cycle), leading to the production of reducing equivalents. NADH/FADH are subsequently oxidized to generate ATP (solid line). In a fasted state, when glucose availability is low, the cell uses alternative ketone bodies that pass into the cell through mono carboxyl ate transporters (MCTs, indicated with a dotted line). Ketone bodies are converted into acetyl-CoA, prompting the citric acid cycle to proceed, similarly producing NADH (+H), which is oxidized to produce ATP (dotted line). (B) Ketogenic diet impact in normal brain cells. Due to the ketogenic dietinduced competition for available glucose, the primary source of acetyl-CoA switches to ketone bodies. These ketone bodies enter the cell via MCTs, leading to the production of acetyl-CoA. This initiates the citric acid cycle (Krebs cycle), resulting in the generation of NADH (+H). NADH is then oxidized, producing ATP.
图 1: (A) 正常脑细胞中葡萄糖和酮体代谢的简化示意图。在无氧条件下,正常细胞在细胞质中进行糖酵解,产生少量但快速的能量。在有氧条件下,正常细胞在线粒体中进行速度较慢但效率更高的氧化磷酸化以产生能量。在进食状态下,细胞能量来源于葡萄糖代谢(用实线表示),通过在细胞质中进行糖酵解形成丙酮酸,随后进入线粒体。在线粒体内,丙酮酸转化为乙酰辅酶A,启动三羧酸循环(Krebs循环),产生还原当量。NADH/FADH随后被氧化生成ATP(实线)。在禁食状态下,当葡萄糖供应不足时,细胞利用通过单羧酸转运体(MCTs,用虚线表示)进入细胞的替代性酮体。酮体转化为乙酰辅酶A,推动三羧酸循环进行,同样产生NADH(+H),其被氧化生成ATP(虚线)。(B) 生酮饮食对正常脑细胞的影响。由于生酮饮食引起对可用葡萄糖的竞争,乙酰辅酶A的主要来源转变为酮体。这些酮体通过MCTs进入细胞,导致乙酰辅酶A的产生。这启动了三羧酸循环(Krebs循环),产生NADH(+H)。NADH随后被氧化,生成ATP。
Each patient underwent a clinical assessment according to the ECOG (Eastern Cooperative Oncology Group) scale, before starting the ketogenic diet and every 3 months after. This scale is intended to assess how a patient’s disease is progressing, how the disease affects the daily living abilities, and to determine appropriate treatment and prognosis. Grade 0 means that the patient is fully active, able to carry pre-disease performance without restriction, while Grade 4 means that the patient is completely disabled; cannot perform any self-care; confined to bed or chair; in Grade 5, the patient is dead. Grades 2 and 3 are intermediary forms (32) (Table 1).
每位患者在开始生酮饮食前及之后每3个月按照ECOG (Eastern Cooperative Oncology Group) 量表进行临床评估。该量表用于评估患者疾病进展程度、疾病对日常生活能力的影响,并确定合适的治疗方案和预后。0级表示患者完全活动自如,能无限制地进行患病前的日常活动;4级表示患者完全丧失活动能力,无法进行任何自理活动,需卧床或坐轮椅;5级表示患者死亡。2级和3级为中间过渡状态 [32] (表 1)。
2.2 Inclusion–exclusion criteria
2.2 纳入-排除标准
Adult patients, 18–75 years old with newly diagnosed glioblastoma multiforme (GBM) were included. Patients with cachexia (weight body ${<}40\mathrm{kg}.$ , with severe cardiovascular or renal disease, and inherited metabolic disorders for which ketogenic diet is contra indicated, were excluded from the study.
纳入标准为18-75岁新确诊的多形性胶质母细胞瘤(GBM)成年患者。排除标准包括恶病质(体重<40kg)、严重心血管或肾脏疾病患者,以及生酮饮食禁忌的遗传性代谢疾病患者。
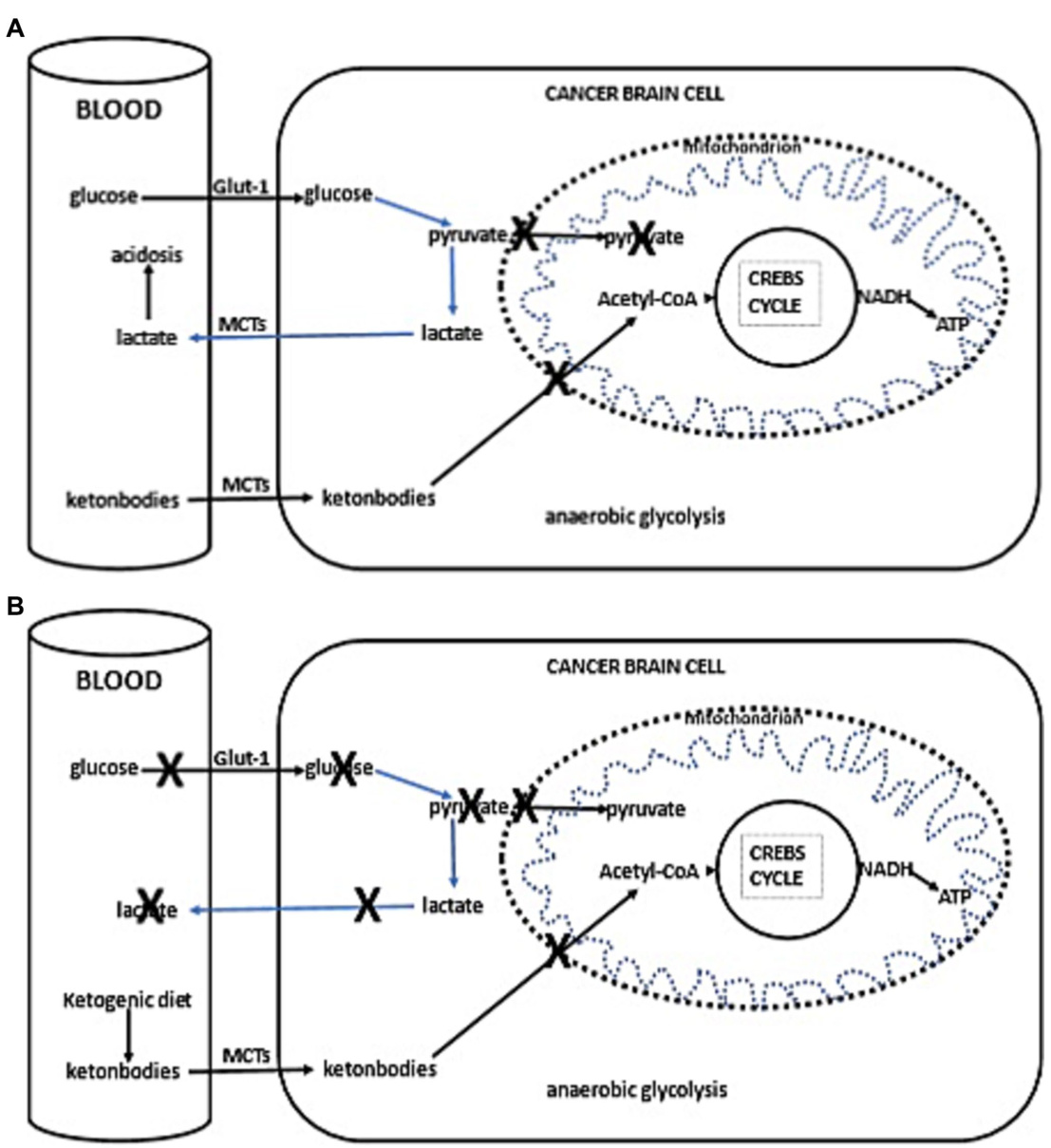
FIGURE 2 (A) Simplified schema of glucose and fat metabolism in a cancer cells. In addition to their reliance on glycolysis, most tumors, including those in the brain, exhibit abnormalities in the number and function of their mitochondria. Functional mitochondria are essential for utilizing ketones as an energy source. Consequently, for malignant cells, glycolysis becomes the primary source of ATP through the Embden–Meyerhof–Parnas pathway, regardless of oxygen availability. This glycolytic process is much less efficient than oxidative phosphor yl ation, as it generates less ATP per glucose molecule metabolized. Thus, conversion of glucose into lactic acid, bypassing oxidative phosphor yl ation, leads to reduced ATP production. To fulfill the elevated energy needs necessary for rapid tumor growth, cancer cells escalate glycolytic activity. The accumulation of lactate in cancer cells promotes lactate transport to the blood and extracellular fluid via proton-linked MCTs. This accumulation of lactic acid contributes to acidosis in both the blood and extracellular spaces, promoting ang io genesis, metastasis, and notably, immunosuppression, which is associated with worse clinical prognosis. (B) Ketogenic diet impact in cancer cells. The adoption of the ketogenic diet leads to an increase in hepatic keto genesis, which in turn inhibits glucose uptake by cells, positioning ketones as the primary energy substrate. However, the reduced ability of cancer cells to oxidize ketones efficiently, compounded by glucose deprivation, may result in reduced proliferation rates.
图 2: (A) 癌细胞中葡萄糖和脂肪代谢的简化示意图。除依赖糖酵解外,大多数肿瘤(包括脑部肿瘤)的线粒体数量和功能均存在异常。功能性线粒体是利用酮体作为能量来源的关键。因此,无论氧气供应是否充足,恶性细胞都主要通过 Embden-Meyerhof-Parnas 途径将糖酵解作为 ATP 的主要来源。与氧化磷酸化相比,该糖酵解过程效率低得多,因为每代谢一个葡萄糖分子产生的 ATP 更少。因此,绕过氧化磷酸化将葡萄糖转化为乳酸会导致 ATP 生成减少。为满足肿瘤快速生长所需的高能量需求,癌细胞会增强糖酵解活性。癌细胞中乳酸的积累会促进乳酸通过质子偶联的 MCTs 转运至血液和细胞外液。这种乳酸积累会导致血液和细胞外空间酸中毒,促进血管生成、转移,尤其是与不良临床预后相关的免疫抑制。(B) 生酮饮食对癌细胞的影响。采用生酮饮食会增加肝脏生酮作用,从而抑制细胞对葡萄糖的摄取,使酮体成为主要能量底物。然而,癌细胞氧化酮体的能力降低,再加上葡萄糖剥夺,可能导致增殖速率下降。
2.3 Ketogenic diet administration
2.3 生酮饮食干预
Following this enrollment evaluation, consultation was done by a clinical dietician and then, if the patient agreed to participate, the ketogenic diet was initiated. Prior to initiating the diet, comprehensive hematological and biochemical testing was conducted. This included, among other evaluations, a full blood count, a complete lipid profile, and purine levels to prevent the risk of gout. We started with a 1:1 diet (fat: protein $^{+}$ carbohydrates), and gradually, while monitoring ketonemia and glycemia, increased the ratio with the goal of reaching a 3:1 ratio, aiming for ketone values $>3.5\mathrm{mM/L}$ and glucose values ${<}80\mathrm{mg/dL}$ . Energy requirements were calculated based on the patients’ body weight at the time we took them on, using the Mifflin-St Jeor equation (which uses the current body weight). Our goal was not weight loss during the phase of standard therapy in these oncology patients However, we did observe that patients on the ketogenic diet initially lost weight and then stabilized. Thus, energy needs were calculated to maintain weight, though as previously mentioned, patients initially lost weight (on average $3{-}5\mathrm{kg}$ ), regardless of minor increases in caloric intake. The highest ketogenic ratio we reached was around 2.5:1 since, in adults, achieving a higher ketogenic ratio while meeting daily protein requirements is challenging; these requirements were calculated to provide $0.95{-}1.2\mathrm{g}/\mathrm{kg}/\mathrm{day}$ . We did not apply caloric restriction for our patients,. Besides, we did not have obese patients, only a few who were overweight. The diet was adapted to Mediterranean diet patterns due to the patient’s aggravated status from the disease itself and standard treatments, with the intention of avoiding additional side effects. The Mediterranean ketogenic diet, unlike the western ketogenic diet that uses saturated fatty acids as a source of fat, is based mainly on olive oil and other mono and polyunsaturated fatty acid sources (olives, avocado, nuts, ω-3 rich fish) and emphasizes the consumption of ω-rich fish and seafood as a source of protein. This dietary pattern is milder than the classic North American-Northwestern. An example of the diet applied is shown in Table 2.
在完成入组评估后,由临床营养师进行咨询。若患者同意参与,则开始生酮饮食。启动饮食方案前,我们进行了全面的血液学和生化检测,包括全血细胞计数、完整血脂谱及嘌呤水平检测以预防痛风风险。
初始采用1:1饮食比例(脂肪:蛋白质$^{+}$碳水化合物),通过监测血酮和血糖水平逐步提高比例,目标达到3:1比例,使血酮值$>3.5\mathrm{mM/L}$且血糖值${<}80\mathrm{mg/dL}$。能量需求根据患者入组时的体重采用Mifflin-St Jeor公式(基于当前体重)计算。
对于这些肿瘤患者的标准治疗阶段,我们的目标并非减重。但观察到生酮饮食患者初期体重下降后趋于稳定。因此尽管能量计算以维持体重为目标,患者初期仍平均减重$3{-}5\mathrm{kg}$(与热量摄入轻微增加无关)。成人因需满足每日$0.95{-}1.2\mathrm{g}/\mathrm{kg}$蛋白质需求,我们达到的最高生酮比例约为2.5:1。
未对患者实施热量限制,且入组患者中仅有少数超重者。考虑到疾病本身和标准治疗导致的健康恶化,饮食方案调整为地中海模式以避免额外副作用。与使用饱和脂肪酸的西方生酮饮食不同,地中海生酮饮食主要以橄榄油等单不饱和/多不饱和脂肪酸(橄榄、牛油果、坚果、富含ω-3的鱼类)为基础,并强调摄入富含ω的鱼类和海鲜作为蛋白质来源。该饮食模式比经典的北美-西北欧方案更温和。具体饮食示例见表2。
TABLE 1 Characteristics of all patients who participated in the study.
| P | G | A | DD | DS-TOO | Chemo + Rad prior KD administration | MB |
| 1 | M | 41 | 29/12/16 | 4/1/17 Total resection | 30 cycles of radiation (02/17) Temozolamide | IDH1(-) |
| 2 | M | 56 | 28/05/20 | 5/6/20 Total resection | 30 cycles of radiation (08/20) Temozolamide | IDH1(-) |
| 3 | F | 64 | 19/04/21 | 26/4/21 Total resection | 30 cycles of radiation (05/21) Temozolamide | IDH1(-) |
| 4 | M | 61 | 22/02/18 | 13/3/18 Stereotactic biopsy | 30 cycles of radiation (04/18) Temozolamide | IDH1(-) |
| 5 | M | 48 | 24/04/20 | 4/5/20 Total resection | 30 cycles of radiation (06/20) Temozolamide | IDH1(-) |
| 6 | M | 58 | 08/05/20 | 20/5/20 Total resection | 30 cycles of radiation (07/20) Temozolamide | IDH 1-2(-) |
| 7 | M | 60 | 11/12/18 | 19/12/18 Subtotal resection | 30 cycles of radiation (02/19) Temozolamide | IDH 1-2 (-) |
| 8 | M | 69 | 15/03/17 | 23/03/17 Total resection | 30 cycles of radiation (05/17) Temozolamide | IDH 1 (-) |
| 9 | M | 53 | 1/10/20 | 15/10/20 Subtotal resection | 30 cycles of radiation (11/20) Temozolamide | IDH 1 (-) |
| 10 | F | 57 | 16/02/21 | 26/2/21 Total resection | 30 cycles of radiation (04/21) Temozolamide | IDH1 (-) |
| 11 | F | 36 | 20/01/18 | 25/11/18 Subtotal resection | 30 cycles of radiation (02/19) Temozolamide | IDH1 (+) |
| 12 | F | 44 | 08/09/17 | 19/9/17 Total resection | 30 cycles of radiation (11/17) Temozolamide | IDH1 (+) |
| 34 | 24/07/20 | 3/8/20 Total resection | 30 cycles of radiation (10/20) Temozolamide | IDH 1-2 (-) | ||
| 16 | ||||||
| 17 | F | 59 | 04/04/19 | |||
| IDH 1 (-) | ||||||
| M | 71 | 22/01/20 | 24/2/20 | 30 cycles of radiation (04/20) | ||
| 14 | ||||||
| F | 59 | |||||
| 08/01/16 | IDH 1-2 (-) | |||||
| 22/1/16 | 30 cycles of radiation (03/16) | |||||
| Subtotal resection | ||||||
| Temozolamide | ||||||
| 15 | M | 51 | 02/05/19 | 16/5/19 | 30 cycles of radiation (06/19) | IDH 1-2 (-) |
| Total resection | Temozolamide | |||||
| F | 75 | 15/07/21 | 29/7/21 | 30 cycles of radiation (09/21) | IDH 1 (-) | |
| Total resection | Temozolamide | |||||
| 8/5/19 | 30 cycles of radiation (06/19) | IDH 1-2 (-) | ||||
| Subtotal resection | Temozolamide | |||||
| 18 |
Patients 1–6 (bold letters) are those who maintained the diet beyond 6 months. P, Patient; G, Gender; A, Age; DD, Diagnosis date; DS-TOO, Date of surgery-type of operation; Chemo $+\mathrm{Rad}$ , Chemotherapy $^+$ Radiation; KD, ketogenic diet; MB, Molecular biology.
表 1: 参与研究的所有患者特征。
| P | G | A | DD | DS-TOO | Chemo + Rad prior KD administration | MB |
|---|---|---|---|---|---|---|
| 1 | M | 41 | 29/12/16 | 4/1/17 Total resection | 30 cycles of radiation (02/17) Temozolamide | IDH1(-) |
| 2 | M | 56 | 28/05/20 | 5/6/20 Total resection | 30 cycles of radiation (08/20) Temozolamide | IDH1(-) |
| 3 | F | 64 | 19/04/21 | 26/4/21 Total resection | 30 cycles of radiation (05/21) Temozolamide | IDH1(-) |
| 4 | M | 61 | 22/02/18 | 13/3/18 Stereotactic biopsy | 30 cycles of radiation (04/18) Temozolamide | IDH1(-) |
| 5 | M | 48 | 24/04/20 | 4/5/20 Total resection | 30 cycles of radiation (06/20) Temozolamide | IDH1(-) |
| 6 | M | 58 | 08/05/20 | 20/5/20 Total resection | 30 cycles of radiation (07/20) Temozolamide | IDH 1-2(-) |
| 7 | M | 60 | 11/12/18 | 19/12/18 Subtotal resection | 30 cycles of radiation (02/19) Temozolamide | IDH 1-2 (-) |
| 8 | M | 69 | 15/03/17 | 23/03/17 Total resection | 30 cycles of radiation (05/17) Temozolamide | IDH 1 (-) |
| 9 | M | 53 | 1/10/20 | 15/10/20 Subtotal resection | 30 cycles of radiation (11/20) Temozolamide | IDH 1 (-) |
| 10 | F | 57 | 16/02/21 | 26/2/21 Total resection | 30 cycles of radiation (04/21) Temozolamide | IDH1 (-) |
| 11 | F | 36 | 20/01/18 | 25/11/18 Subtotal resection | 30 cycles of radiation (02/19) Temozolamide | IDH1 (+) |
| 12 | F | 44 | 08/09/17 | 19/9/17 Total resection | 30 cycles of radiation (11/17) Temozolamide | IDH1 (+) |
| 34 | 24/07/20 | 3/8/20 Total resection | 30 cycles of radiation (10/20) Temozolamide | IDH 1-2 (-) | ||
| 16 | ||||||
| 17 | F | 59 | 04/04/19 | IDH 1 (-) | ||
| M | 71 | 22/01/20 | 24/2/20 | 30 cycles of radiation (04/20) | ||
| 14 | ||||||
| F | 59 | 08/01/16 | 22/1/16 Subtotal resection | 30 cycles of radiation (03/16) Temozolamide | IDH 1-2 (-) | |
| 15 | M | 51 | 02/05/19 | 16/5/19 Total resection | 30 cycles of radiation (06/19) Temozolamide | IDH 1-2 (-) |
| F | 75 | 15/07/21 | 29/7/21 Total resection | 30 cycles of radiation (09/21) Temozolamide | IDH 1 (-) | |
| 8/5/19 Subtotal resection | 30 cycles of radiation (06/19) Temozolamide | IDH 1-2 (-) |
患者1-6(加粗字体)为坚持生酮饮食超过6个月者。P,患者编号;G,性别;A,年龄;DD,确诊日期;DS-TOO,手术日期-手术类型;Chemo + Rad,化疗联合放疗;KD,生酮饮食;MB,分子生物学特征。
2.4 Assessment criterion: statistics
2.4 评估标准:统计
As an assessment criterion, we set an optimistic target for adherence to the ketogenic diet beyond 6 months. The six-month timeframe was arbitrarily chosen based on our previous experience with studies involving the implementation of the ketogenic diet, where it served as an indicator of adherence (33, 34). We considered the therapeutic combination successful if the survival from diagnosis reached at least 3 years. The patients self-measured their blood glucose and ketone levels with blood glucose and $\beta$ -ketone test strips, in the morning and afternoon prep rand i ally. Initially, they did this daily for the first month and then twice a week. The readings were kept and referred to the dietitian with the diet records.
作为评估标准,我们设定了一个乐观目标:生酮饮食坚持超过6个月。选择六个月时间框架是基于我们以往实施生酮饮食研究的经验,该时长曾被用作依从性指标 [33, 34]。若患者从确诊起存活至少3年,我们则认为这种治疗组合是成功的。患者使用血糖和$\beta$-酮体试纸自行测量晨间及午后的血糖与酮体水平,最初第一个月每日监测,之后改为每周两次。所有读数记录均与饮食日志一并保存并提交给营养师参考。
TABLE 2 Example of ketogenic diet 2,150 Kcal 2:1 ratio.
| Morning | Snack | Lunch | Snack | Dinner |
| 17g tuna in oil | 20gfetacheese | 93g sardines | 1eggfortifiedwitho-3fatty acids | 117 g raw green salad |
| 60gketo-focaccia | 8golive oil | 41 g olive oil | 15g avocado | 93g salmon |
| 15 g tahini | 21 g olives | 117gboiledgreens | 9 g olive oil | 41 g olive oil |
| 14 g olive oil | 25g melon | |||
| 30g avocado |
Daily menu plan.
表 2: 生酮饮食示例 2,150 大卡 2:1 比例
| 早晨 | 零食 | 午餐 | 零食 | 晚餐 |
|---|---|---|---|---|
| 17克油浸金枪鱼 | 20克羊奶酪 | 93克沙丁鱼 | 1个富含ω-3脂肪酸的鸡蛋 | 117克生绿色沙拉 |
| 60克生酮佛卡夏 | 8克橄榄油 | 41克橄榄油 | 15克牛油果 | 93克三文鱼 |
| 15克芝麻酱 | 21克橄榄 | 117克水煮青菜 | 9克橄榄油 | 41克橄榄油 |
| 14克橄榄油 | 25克甜瓜 | |||
| 30克牛油果 |
每日菜单计划
Statistical analysis was done using the Statistical Analysis Systems statistical software package, version 20 (SAS Institute). Results were regarded as significant when $p<0.05$ . The difference between the two groups was assessed by the $t.$ -test for paired comparisons.
统计分析采用统计分析系统(Statistical Analysis Systems)统计软件包20版(SAS Institute)完成。当 $p<0.05$ 时认为结果具有统计学意义。两组间差异通过配对比较的 $t.$ 检验进行评估。
3 Cases presentation
3 案例展示
3.1 Patient 1: 84-months follow-up
3.1 病例1:84个月随访
A 41-year-old man was diagnosed with GBM of the left temporal lobe on December 2016 following a brain MRI (Figure 3). The presenting symptom was persistent headache and gradual wordfinding difficulty (anomic aphasia). He underwent surgical resection in January 2017, followed by 30 sessions of radiotherapy $(60\mathrm{Gy})$ along with chemotherapy (temozolomide). His to pathological examination was typical of GBM with IDH1-negative/MGMT-nonmethylated (Figure 4). On March 2017, the patient was started on a calorie restricted 1.4:1 ketogenic diet as adjunctive therapy (Table 3). Blood ketones and glucose levels were self-monitored daily. The patient achieved adequate ketosis during the first week of instituting the ketogenic diet and maintained high ketone levels $\left(3{-}4\mathrm{mmol/L}\right)$ and adequate blood glucose levels $\mathrm{(60-90~mg/dL)}$ ) throughout the observational period. The ketogenic diet was well tolerated, with only mild gastrointestinal side effects (constipation). In addition, he received temozolomide, initially dosed at $100\mathrm{mg/m^{2}/d a y}$ every other week. After 1 month, his dose increased to $200\mathrm{mg/m^{2}/d a y}$ on the same schedule, without toxicities. Temozolomide maintenance treatment lasted for 2 years. Serial MRI brain imaging was obtained every 4 months. His follow-up brain MRI 79 months after diagnosis shows no evidence of tumor recurrence (Figure 3). The patient reports a residual mild anomia and is currently working as a teacher. His most recent ECOG grade is 0.
一名41岁男性患者于2016年12月经脑部MRI (图3) 确诊为左颞叶胶质母细胞瘤 (GBM) 。主要症状为持续性头痛和渐进性命名障碍 (命名性失语) 。2017年1月接受手术切除,随后进行30次放疗 $(60\mathrm{Gy})$ 联合替莫唑胺化疗。病理检查显示典型IDH1阴性/MGMT非甲基化的GBM特征 (图4) 。2017年3月起,患者开始采用1.4:1热量限制生酮饮食作为辅助治疗 (表3) ,每日自行监测血酮和血糖水平。实施生酮饮食首周即达到理想酮症状态,并在整个观察期间维持较高酮体水平 $\left(3{-}4\mathrm{mmol/L}\right)$ 和正常血糖范围 $\mathrm{(60-90~mg/dL)}$ 。该饮食方案耐受性良好,仅出现轻微胃肠道副作用 (便秘) 。同步接受替莫唑胺治疗,初始剂量为隔周 $100\mathrm{mg/m^{2}/d a y}$ ,1个月后按相同方案增至 $200\mathrm{mg/m^{2}/d a y}$ ,未出现毒性反应。替莫唑胺维持治疗持续2年,每4个月进行系列脑部MRI复查。确诊79个月后的随访MRI显示无肿瘤复发迹象 (图3) 。患者目前遗留轻度命名障碍,从事教师职业,最新ECOG评分为0级。
3.2 Patient 2: 43-months follow-up
3.2 病例2:43个月随访
Patient 2 is a 59-year-old male. He is a primary school teacher in very good general condition, with a lean and athletic build (marathon runner). On 23/05/2020, at the age of 56, he was hospitalized due to persistent headache and a sudden onset of left hemi pares is, confusion, and vomiting. Brain CT showed a space-occupying right parietal– temporal lesion with solid and cystic elements and peripheral enhancement, as well as large perifocal edema with midline shift of $12\mathrm{mm}$ . A subsequent brain MRI (Figure 5) showed a large heterogeneous mass in the right tempor o parietal area, $53\mathrm{mm}$ in diameter, with hemorrhagic and necrotic elements and peripheral gadolinium enhancement, surrounded by extensive vasogenic edema. These radiological features were highly suggestive for GBM. On $05/06/2020$ , he underwent a total resection of the tumor through a right temporal craniotomy. He was discharged with le vet i race tam $1{,}000~\mathrm{mg}$ $\times2/$ day and methyl pre dni sol one per os in gradual tapering. His to logical examination (Figure 4) confirmed GBM, immuno his to chemically negative for IDH-1 mutation (GBM NOS). On 28/06/2020 he suffered a lower extremity deep vein thrombosis (DVT) and was placed on a therapeutic dose of heparin for 8 months. The patient followed 30 cycles of radiation (21/7/2020–31/8/2020) and was placed on temozolomide $150:\mathrm{mg/m}^{2}$ , 5 days/month, which continues until the present day. A classic ketogenic diet was implemented on 19/8/2020 with a ketogenic ratio $>2{:}1$ and a total daily calorie intake of $2,150\mathrm{kcal}$ (Table 3).
患者2为59岁男性,小学教师,总体健康状况良好,体型精瘦且运动型(马拉松跑者)。2020年5月23日(56岁时)因持续头痛伴突发左侧偏瘫、意识混乱及呕吐入院。脑部CT显示右顶颞叶占位性病变,含实性与囊性成分及周边强化,伴大面积瘤周水肿(中线移位达12mm)。后续脑部MRI(图5)显示右颞顶区存在53mm直径的异质性肿块,含出血坏死成分及周边钆剂强化,周围伴广泛血管源性水肿,影像学特征高度提示胶质母细胞瘤(GBM)。2020年6月5日行右颞开颅肿瘤全切术,出院时服用左乙拉西坦1,000mg(每日两次)并逐步减量口服甲泼尼龙。组织学检查(图4)确诊为IDH-1突变阴性的GBM(非特指型)。2020年6月28日出现下肢深静脉血栓(DVT),接受治疗剂量肝素治疗8个月。患者完成30次放疗(2020年7月21日–8月31日)后持续服用替莫唑胺(150mg/m²,每月5天)至今。2020年8月19日开始实施经典生酮饮食(生酮比>2:1,每日总热量摄入2,150kcal)(表3)。
The patient achieved satisfactory ketosis as early as the 1st week of diet initiation, with blood ketone levels of $2.9{-}5~\mathrm{mmol/L}$ and morning blood sugar of approximately $72{-}75\mathrm{mg/dL}$ . Nutritional ketosis is maintained until today. Close monitoring with serial MRIs shows no evidence of GBM recurrence throughout the observation period of 43 months (Figure 5). The patient is fully ambulatory, still working as a primary school teacher, with no imaging or clinical signs of disease activity. His most recent ECOG grade is 0.
患者在开始饮食治疗的第1周就达到了理想的酮症状态,血酮水平为$2.9{-}5~\mathrm{mmol/L}$,晨间血糖约为$72{-}75\mathrm{mg/dL}$。营养性酮症状态持续至今。通过连续MRI密切监测显示,在43个月的观察期内未出现胶质母细胞瘤(GBM)复发迹象(图5)。患者行动完全自如,仍担任小学教师工作,影像学及临床表现均无疾病活动征象。其最近一次ECOG评分为0级。
3.3 Patient 3: 33 months follow-up
3.3 患者3:33个月随访
A 65-year-old female was diagnosed with GBM in April 2021. Her presenting symptom was anomic aphasia. Brain MRI was remarkable for a space-occupying lesion at the left temporal lobe with he t erogenous signal on T2 sequences and irregular enhancement surrounded by large vasogenic oedema, consistent with GBM (Figure 6). Her his to pathological examination confirmed IDH1-negative GBM (Figure 4). She underwent surgery (total resection of the tumor) followed by radiotherapy—a total of 60 Gy in 30 RTs - and maintenance chemotherapy with temozolomide $120\mathrm{mg/m^{2}/d a y,}$ for 5 days each month. Ketogenic diet with a ketogenic ratio of $>2:1$ and total daily calorie intake of 1,500 kcal/day was introduced in July of the same year (Table 3). After 6 months the patient decided to abandon the diet due to perceived dietary restrictive ness. On April 2022, 4 months after KD discontinuation, GBM recurrence was followed by stereo tactic radio surgery (CyberKnife) (Figure 6). She also initiated second line chemotherapy with bevacizumab $(5{-}7\mathrm{mg/kg)}$ and irinotecan $120\mathrm{mg/}$ $\mathrm{m}^{2}$ twice/month, which is maintained until the present day. After relapse, the patient agreed to reinitiate the diet with improved adherence. Blood glucose levels were maintained between 75 and $85\mathrm{mg/dL}$ and ketone levels between 2 and 3 mmol/L. Her present ECOG grade is 0.
一名65岁女性于2021年4月被诊断为胶质母细胞瘤(GBM),首发症状为命名性失语。脑部MRI显示左颞叶占位性病变,T2序列呈不均匀信号伴不规则强化及周围大面积血管源性水肿,符合GBM表现(图6)。组织病理学检查确诊为IDH1阴性GBM(图4)。患者接受肿瘤全切手术后,进行了30次总剂量60Gy的放疗,并采用替莫唑胺维持化疗(120mg/m²/天,每月连用5天)。同年7月开始生酮饮食(生酮比>2:1,每日总热量摄入1500kcal)(表3)。6个月后患者因感觉饮食限制过多而中止该方案。2022年4月(停用生酮饮食4个月后)肿瘤复发,遂接受立体定向放射外科治疗(CyberKnife)(图6),并开始二线化疗方案:贝伐珠单抗(5-7mg/kg)联合伊立替康(120mg/m²,每月两次),该方案持续至今。复发后患者同意重新开始生酮饮食且依从性提高,血糖维持在75-85mg/dL,血酮水平维持在2-3mmol/L。目前ECOG评分为0分。
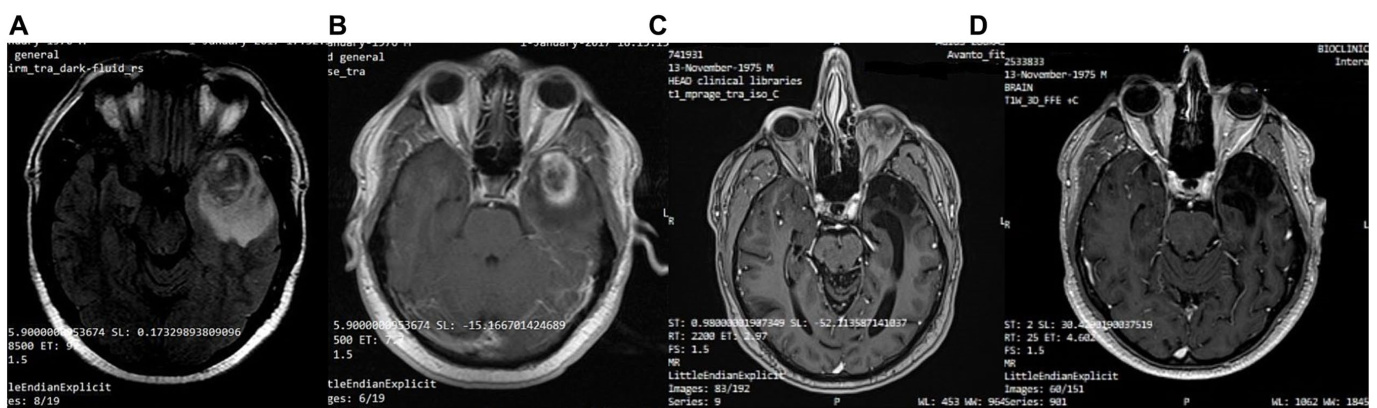
FIGURE 3 Patient 1: (A) Pre-operative brain MRI (T2/FLAIR) (B) Pre-operative brain MRI (T1 with contrast) (C) 38-month follow-up brain MRI (T1 with contrast) (D) 80-month follow-up brain MRI (T1 with contrast).
图 3: 患者1: (A) 术前脑部MRI (T2/FLAIR) (B) 术前脑部MRI (T1增强) (C) 38个月随访脑部MRI (T1增强) (D) 80个月随访脑部MRI (T1增强)。
3.4 Patient 4: 43-month follow-up
3.4 病例4:43个月随访
A 63-year-old man was diagnosed in February 2018 with GBM following a first epileptic seizure. The patient experienced an episode of focal seizures involving the right upper and lower limb. On neurological examination, a mild right-sided hemi pares is was noted. Brain MRI revealed a large heterogeneous ly enhancing tumor in the left parietal lobe with a central nodule and surrounding vasogenic oedema, extending through the corpus callosum to the contra lateral side (Figure 7). An EEG demonstrated paroxysmal activity consisting of intermittent sharp theta waves on the left fronto central region and the patient was put on le vet i race tam. Stereo tactic brain biopsy and his to pathological examination confirmed IDH1-negative GBM (Figure 4). The patient was considered inoperable due to the extent of the tumor, which infiltrated the corpus callosum and extended across the midline. The patient received radiotherapy (30 sessions, 60 Gy in total) along with chemotherapy (temozolomide) and corticosteroids (dex amet has one per os). A 3:1 ketogenic diet was initiated in April 2018 just before radiotherapy (Table 3). After radiation therapy, corticosteroids were gradually withdrawn and the patient was maintained on a treatment regimen of $250\mathrm{mg}$ temozolomide in total per day for 5 days every 4 weeks (until July 2019), alongside ketogenic diet therapy. The diet was well-tolerated, and the patient achieved and maintained ketosis (ketonemia $3{-}4\mathrm{mmol/L}$ ) and lower blood glucose levels $(70{-}85\mathrm{mg/dL})$ throughout the follow-up period. Brain MRI was performed every 3 months showing no tumor progression and less surrounding vasogenic oedema, while the enhancing nodule in the left semioval center was decreasing with minor enhancement (Figure 7).
2018年2月,一名63岁男性首次癫痫发作后被诊断为胶质母细胞瘤(GBM)。患者出现累及右上肢和右下肢的局灶性癫痫发作。神经系统检查发现轻度右侧偏瘫。脑部MRI显示左顶叶存在一个大型异质性强化肿瘤,中央有结节并伴有周围血管源性水肿,肿瘤通过胼胝体延伸至对侧 (图7)。脑电图显示左额中央区出现阵发性间歇性尖θ波活动,患者开始服用左乙拉西坦。立体定向脑活检及组织病理学检查确诊为IDH1阴性胶质母细胞瘤 (图4)。由于肿瘤范围广泛,已浸润胼胝体并跨越中线,患者被判定为无法手术。患者接受了放疗(30次,总剂量60Gy)联合化疗(替莫唑胺)及皮质类固醇治疗(口服地塞米松)。2018年4月放疗前开始采用3:1生酮饮食 (表3)。放疗结束后逐渐停用皮质类固醇,并维持每4周服用5天(每日总量$250\mathrm{mg}$)替莫唑胺的治疗方案(持续至2019年7月),同时配合生酮饮食疗法。患者对饮食方案耐受良好,在整个随访期间保持酮症状态(血酮$3{-}4\mathrm{mmol/L}$)及较低血糖水平$(70{-}85\mathrm{mg/dL})$。每3个月进行的脑部MRI显示肿瘤无进展且周围血管源性水肿减轻,同时左半卵圆中心强化结节逐渐缩小且强化程度减弱 (图7)。
However, 32 months after diagnosis, the patient suffered a GBM relapse (Figure 7). While continuing the ketogenic diet, he underwent surgical resection and was put on second line chemotherapy with bevacizumab $(5{-}7\mathrm{mg/kg})$ and irinotecan $\mathrm{120~mg/m^{2}},$ ) until his death in September 2021 (43 months post-diagnosis).
然而,确诊32个月后,患者出现胶质母细胞瘤复发 (图 7)。在坚持生酮饮食的同时,他接受了手术切除,并开始使用贝伐珠单抗 $(5{-}7\mathrm{mg/kg})$ 和伊立替康 $\mathrm{120~mg/m^{2}}$ 进行二线化疗,直至2021年9月去世 (确诊后43个月)。
3.5 Patient 5: 44 months follow-up
3.5 患者5:44个月随访
A 48-year-old male guitar teacher presented at the emergency department in April 2020 with confusion, agitation, anomic aphasia, and left hemi pares is. His symptoms developed gradually over a period of 1 month, during which he complained of frequent headaches. Brain
一名48岁男性吉他教师于2020年4月因意识模糊、躁动、命名性失语及左侧轻偏瘫就诊急诊科。症状在1个月内逐渐发展,期间主诉频繁头痛。脑部
MRI demonstrated a large contrast enhancing right hemispheric lesion expanding contra laterally through corpus callosum, as well as supra ten tori ally to the right cerebellum, with irregular borders, necrotic and hemorrhagic areas and vast peri lesion al vasogenic oedema (Figure 8). These features were consistent with GBM, as was later confirmed by his to pathological examination that showed grade IV IDH1-negative GBM (Figure 4). Ketogenic diet was introduced soon after diagnosis, as an adjunctive treatment to standard of care (tumor resection, radiotherapy, chemotherapy). Anti convulsive treatment (le vet i race tam) was also started due to focal aware motor seizures. The patient was put on classic ketogenic diet (ketogenic ratio of 2.5:1) with MCT supplement ation, with a total caloric intake of 2000 kcal/day, containing $189\mathrm{g/d}$ fat, $65~\mathrm{g/d}$ proteins, $11\mathrm{g}/\mathrm{d}$ carbohydrates and $_{3-4\mathrm{g}}$ MCT per meal (Table 3). The patient achieved and maintained ketosis (ketone levels $3{-}4\mathrm{mmol/L}$ ) and lower blood glucose levels $(70{-}85\mathrm{mg/dL})$ ) soon after KD initiation. As a side effect during KD, the patient reported chronic constipation that was relieved by conservative or p harm a co logical means. During follow-up, blood ketone levels oscillated between $2{-}3\mathrm{mmol/L}$ and blood glucose ranged from 80 to $90\mathrm{mg/dL}$ .
MRI显示右侧大脑半球有一处大型对比增强病灶,通过胼胝体向对侧扩展,并向上累及右侧小脑幕,边界不规则,伴有坏死、出血区域及广泛的病灶周围血管源性水肿 (图 8)。这些特征符合胶质母细胞瘤 (GBM) 表现,后续组织病理学检查证实为IDH1阴性IV级GBM (图 4)。确诊后立即在标准治疗(肿瘤切除、放疗、化疗)基础上引入生酮饮食作为辅助疗法。由于出现局灶性意识清醒型运动性癫痫发作,同时开始抗惊厥治疗(左乙拉西坦)。患者采用经典生酮饮食(生酮比例2.5:1)并补充中链甘油三酯(MCT),每日总热量摄入2000千卡,包含脂肪189g/d、蛋白质65g/d、碳水化合物11g/d,每餐添加3-4g MCT (表 3)。开始生酮饮食后患者迅速达到并维持酮症(酮体水平3-4mmol/L),血糖水平降低至70-85mg/dL。生酮期间出现慢性便秘副作用,通过保守或药物手段缓解。随访期间血酮水平波动于2-3mmol/L,血糖维持在80-90mg/dL范围。
The patient had no evidence of disease progression for almost 3 years, having a mild residual left hemi pares is and therefore being able to carry lighter everyday activities (ECOG grade 1). However, in May 2023 (36 months post diagnosis), the patient suffered a GBM recurrence (Figure 8) and was initiated with second line bevacizumab $(5{-}7\mathrm{mg/kg)}$ and irinotecan $(120\mathrm{mg/m}^{2^{*}}$ ) every other week. He is currently under CyberKnife treatment (4–5 sessions over 1–2 weeks). His present ECOG performance status is 3 (capable of only limited self-care; confined to bed or chair more than $50%$ of waking hours).
该患者在近3年内未出现疾病进展迹象,仅遗留轻度左侧偏瘫,因此能够从事较轻的日常活动 (ECOG 1级)。然而在2023年5月 (确诊后36个月),患者出现胶质母细胞瘤复发 (图8),开始接受二线贝伐珠单抗 $(5{-}7\mathrm{mg/kg})$ 联合伊立替康 $(120\mathrm{mg/m}^{2^{*}}$) 隔周治疗。目前正在接受射波刀治疗 (1-2周内4-5次疗程),现ECOG体能状态评分为3级 (仅能有限自理;清醒状态下超过 $50%$ 时间需卧床或坐椅)。
3.6 Patient 6: 36 months follow-up
3.6 病例6:36个月随访
A 58-year-old priest presented with neuro-psychiatric symptoms in April 2020, such as headaches, personality changes and aggressiveness. For this reason, he underwent a brain MRI, which revealed a space-occupying lesion on the left occipito-parietal region with heterogeneous enhancement (Figure 9). The patient underwent total excision of the tumor on May 20, 2020. His to logical examination demonstrated GBM immuno his to chemically negative for IDH-1&2 mutation (GBM
一位58岁的神职人员于2020年4月出现神经精神症状,包括头痛、性格改变和攻击性。为此,他接受了脑部MRI检查,结果显示左侧枕顶区存在不均匀强化的占位性病变(图9)。患者于2020年5月20日接受了肿瘤全切术。组织学检查显示为胶质母细胞瘤(GBM),免疫组化检测IDH-1&2突变呈阴性。
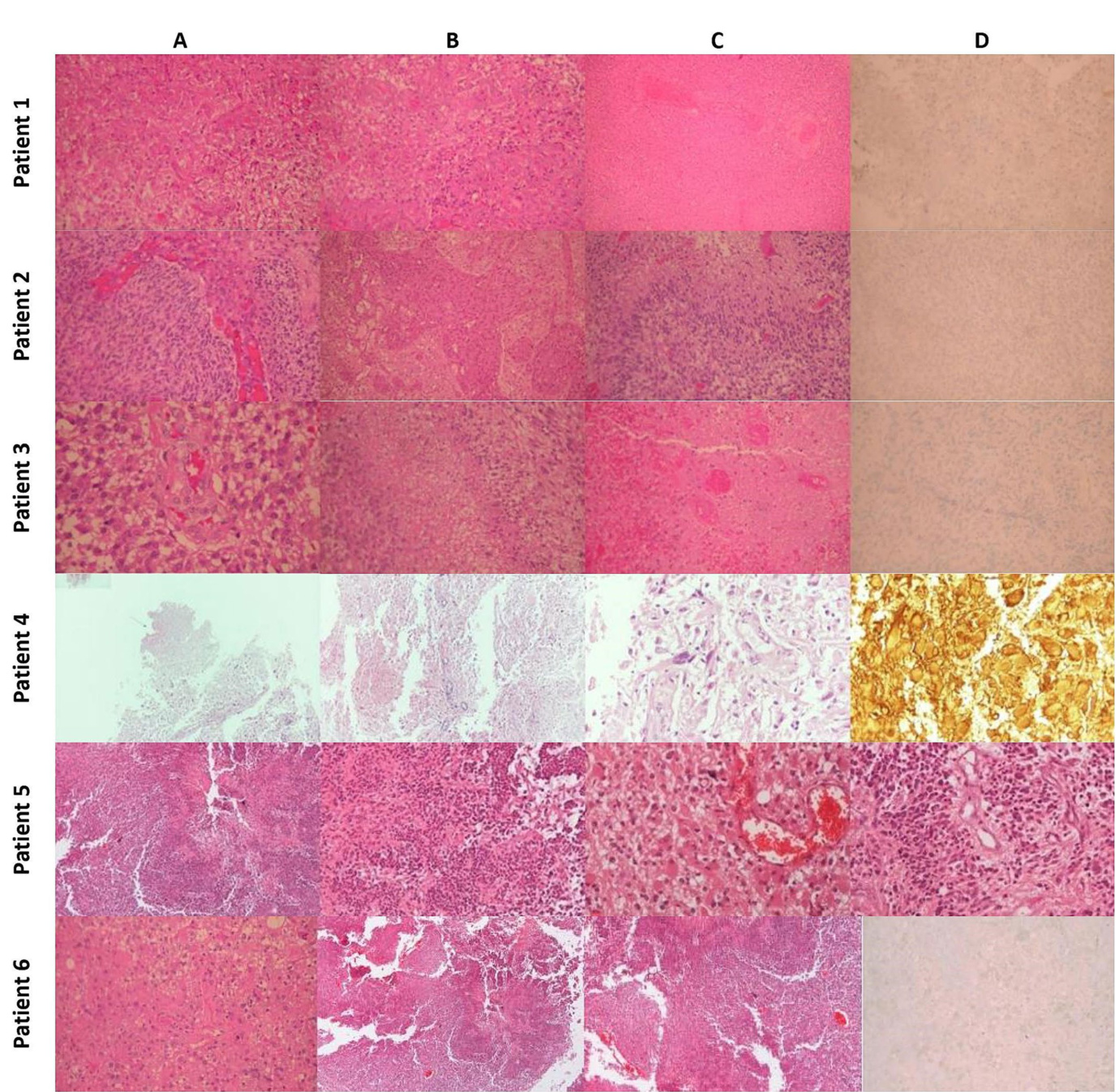
His to pathology: Patient 1 (A) Typical Morphology of Glioblastoma (H&E x200). (B) Significant endo the li al hyperplasia (H&E x200). (C) Extensive Coa gul at ive Necrosis with Thrombotic Vessels (H&E x100). (D) Tumor cells negative for IDH-1 Mutant (IHC x200). Patient 2: (A) Typical morphology of glioblastoma with endo the li al hyperplasia (H&E x200). (B) Severe endo the li al hyperplasia (X100). (C) Palisading necrosis (H&E x200). (D) Tumor cells are negative for IDH-1 mutant (IHC $\times200$ ). Patient 3 (A) Typical morphology of glioblastoma with endo the li al hyperplasia (H&E x200). (B) Palisading necrosis (H&E $\times200,$ ). (C) Extensive coa gul at ive necrosis with thrombotic vessels (H&E $\times200)$ . (D) Tumor cells are negative for IDH-1 mutant (IHC x200). Patient 4 (A) Region of tumor necrosis (H&Ex40). (B) Endo the li al hyperplasia (H&Ex100). (C) Ple om orphic glial cells (H&E $\times400;$ . (D) GFAP $^+$ neoplastic cells and gem is to cyte s (H&Ex400). Patient 5 (A) Region of tumor necrosis (H&E $\times40)$ ). (B) Neoplastic cells surrounding central necrosis (H&E x100). (C) Glial cells with gem is to cy tic features and micro vascular proliferation (H&E $\times200$ ). (D) Endo the li al hyperplasia and micro vascular proliferation (H&E $\times200)$ ). Patient 6 (A) Typical morphology of glioblastoma (H&E $\times200.$ ). (B) Area of geographic necrosis (H&E $\times200$ ). (C) Endo the li al hyperplasia (H&E $\times200)$ . (D) Tumor cells negative for IDH-1 Mutant (IHC $\times200,$ ).

病例1组织病理学: (A) 胶质母细胞瘤典型形态 (H&E ×200)。 (B) 显著内皮增生 (H&E ×200)。 (C) 广泛凝固性坏死伴血栓形成血管 (H&E ×100)。 (D) IDH-1突变阴性肿瘤细胞 (IHC ×200)。
病例2: (A) 伴内皮增生的胶质母细胞瘤典型形态 (H&E ×200)。 (B) 重度内皮增生 (×100)。 (C) 栅栏状坏死 (H&E ×200)。 (D) IDH-1突变阴性肿瘤细胞 (IHC ×200)。
病例3: (A) 伴内皮增生的胶质母细胞瘤典型形态 (H&E ×200)。 (B) 栅栏状坏死 (H&E ×200)。 (C) 广泛凝固性坏死伴血栓形成血管 (H&E ×200)。 (D) IDH-1突变阴性肿瘤细胞 (IHC ×200)。
病例4: (A) 肿瘤坏死区域 (H&E ×40)。 (B) 内皮增生 (H&E ×100)。 (C) 多形性胶质细胞 (H&E ×400)。 (D) GFAP⁺肿瘤细胞与双核细胞 (H&E ×400)。
病例5: (A) 肿瘤坏死区域 (H&E ×40)。 (B) 中央坏死周围肿瘤细胞 (H&E ×100)。 (C) 具双核特征的胶质细胞伴微血管增生 (H&E ×200)。 (D) 内皮增生与微血管增生 (H&E ×200)。
病例6: (A) 胶质母细胞瘤典型形态 (H&E ×200)。 (B) 地图状坏死区域 (H&E ×200)。 (C) 内皮增生 (H&E ×200)。 (D) IDH-1突变阴性肿瘤细胞 (IHC ×200)。
NOS). The patient then underwent 30 sessions of radiation therapy $(60\mathrm{Gy})$ in combination with temozolomide $(75~\mathrm{mg}/\mathrm{m}^{2})$ and dex amet has one. Subsequently, the patient received maintenance chemotherapy with temozolomide $150:\mathrm{mg/m}^{2}$ for 21 months (Figure 9). In November 2020, 6 months after the diagnosis, he started a classical ketogenic diet with a ketogenic ratio $>2.5{:}1$ (Table 3), which was well-tolerated, and the patient maintained low blood glucose levels $(75{-}90~\mathrm{mg/dL})$ and satisfactory ketosis.
随后,患者接受了30次放疗 $(60\mathrm{Gy})$ 联合替莫唑胺 $(75~\mathrm{mg}/\mathrm{m}^{2})$ 和地塞米松治疗。接着,患者继续接受替莫唑胺 $150:\mathrm{mg/m}^{2}$ 维持化疗21个月 (图 9)。2020年11月(确诊后6个月),他开始采用生酮比例 $>2.5{:}1$ 的标准生酮饮食 (表 3),耐受性良好,血糖水平维持在 $(75{-}90~\mathrm{mg/dL})$ 且酮症状态理想。
In February 2022, 22 months after the diagnosis, the patient experienced a focal epileptic seizure and was diagnosed with a recurrence of the GBM on a subsequent brain MRI (Figure 9). For this reason, he underwent 3 sessions of stereo tactic radiotherapy (CyberKnife), and the patient was placed on second-line chemotherapy with bevacizumab $(5{-}7\mathrm{mg/kg})$ and irinotecan $120~\mathrm{mg/}$ $\mathrm{m}^{2}$ twice a month. He continued the ketogenic diet but ultimately passed away from disease complications in May 2023, 36 months after the diagnosis.
2022年2月,即确诊22个月后,患者出现局灶性癫痫发作,后续脑部MRI检查确诊胶质母细胞瘤(GBM)复发(图9)。为此,他接受了3次立体定向放射治疗(CyberKnife),并开始二线化疗方案:贝伐珠单抗$(5{-}7\mathrm{mg/kg})$联合伊立替康$120~\mathrm{mg/}$$\mathrm{m}^{2}$,每两周给药一次。患者持续生酮饮食治疗,但最终于2023年5月因疾病并发症去世,距确诊36个月。
TABLE 3 Patient’s Ketogenic Diet: Total daily intake.
| Patient-KD | Duration | Kcal | Fat g/d | Protein g/d | CHOs g/d | KR |
| Patient 1 Modified Ketogenic Diet | 25.4.2017-present date | 2000 | 169 | 100 | 20 | 1.4: 1 |
| Patient 2 | 1st diet (19.8.2020) | 2,150 | 189.2 | 73.9 | 19.6 | >2: 1 |
| Mediterranean ketogenicdiet with MCTs* | 2nd diet (02.11.2021) | 2,195 | 201.7 | 73.9 | 19.6 | >2,2: 1 |
| 3rd diet | 2,298 | 213 | 75 | 20 | >2,2: 1 | |
| 05.7.2021 - present date 1,500 136 g/d 57 g/d 13 g/d | ||||||
| Mediterranean Ketogenic Diet | ||||||
| Patient 4. Mediterraneanketogenicdiet with | 1st diet (25.4.2018) | 2,000 | 181.9 | 73 | 17.6 | 2:1 |
| MCTs*. | 2nd diet (20.09.2019) | 2,125 | 195.5 | 73.2 | 18 | >2:1 |
| *The patient received >5gr of MCT oil before bedtime. | ||||||
| Patient 5 Mediterranean Ketogenic Diet | 27.4.2020 - present date | 2,000 | 189 g/d | P/8 s9 | 11 g/d | 2.5: 1 |
| Patient 6 | 08.11.2020 05.05.2023 | 2,000 | 166 g/d | 75 g/d | 25 g/d | >2.5: 1 |
Fats are based mainly on olive oil, avocado, olives, raw nuts and seeds and proteins derive mainly from fatty fish and chicken. MCTs, Medium-chain triglycerides; CHOs, Carbohydrates; KD, Ketogenic diet; KR, Ketogenic ratio.
表 3: 患者生酮饮食每日总摄入量
| 患者-KD | 持续时间 | 千卡 | 脂肪(克/天) | 蛋白质(克/天) | 碳水化合物(克/天) | 生酮比 |
|---|---|---|---|---|---|---|
| 患者1改良生酮饮食 | 2017年4月25日至今 | 2000 | 169 | 100 | 20 | 1.4:1 |
| 患者2 | 第一次饮食(2020年8月19日) | 2150 | 189.2 | 73.9 | 19.6 | >2:1 |
| 含MCTs*的地中海生酮饮食 | 第二次饮食(2021年11月2日) | 2195 | 201.7 | 73.9 | 19.6 | >2.2:1 |
| 第三次饮食 | 2298 | 213 | 75 | 20 | >2.2:1 | |
| 地中海生酮饮食 | 2021年7月5日至今 | 1500 | 136 | 57 | 13 | |
| 患者4. 含MCTs*的地中海生酮饮食 | 第一次饮食(2018年4月25日) | 2000 | 181.9 | 73 | 17.6 | 2:1 |
| 第二次饮食(2019年9月20日) | 2125 | 195.5 | 73.2 | 18 | >2:1 | |
| 患者5地中海生酮饮食 | 2020年4月27日至今 | 2000 | 189 | P/8 s9 | 11 | 2.5:1 |
| 患者6 | 2020年11月8日-2023年5月5日 | 2000 | 166 | 75 | 25 | >2.5:1 |
*患者睡前服用>5克MCT油。
脂肪主要来自橄榄油、牛油果、橄榄、生坚果和种子,蛋白质主要来自多脂鱼类和鸡肉。MCTs:中链甘油三酯;CHOs:碳水化合物;KD:生酮饮食;KR:生酮比。
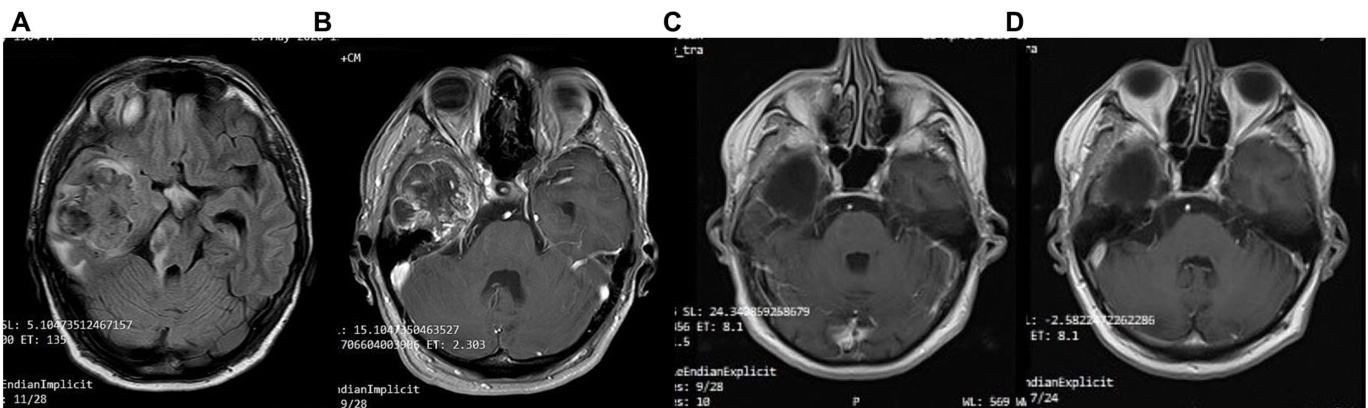
FIGURE 5 Patient 2: (A) pre-operative brain MRI (T2/FLAIR) (B) pre-operative brain MRI (T1 with contrast) (C) 20-month follow-up brain MRI (T1 with contrast) (D) 40-month follow-up brain MRI (T1 with contrast).
图 5 患者 2: (A) 术前脑部 MRI (T2/FLAIR) (B) 术前脑部 MRI (T1 增强) (C) 20 个月随访脑部 MRI (T1 增强) (D) 40 个月随访脑部 MRI (T1 增强)。
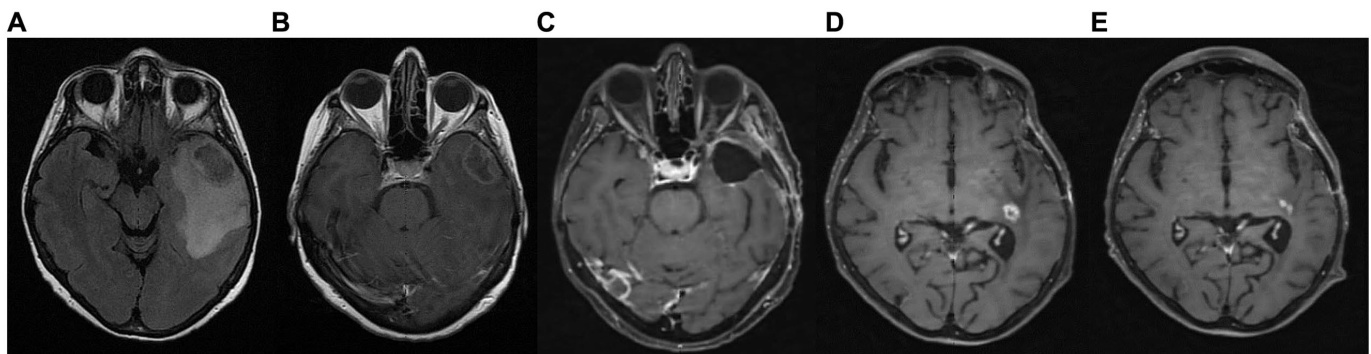
FIGURE 6 Patient 3: (A) brain MRI on diagnosis (T2/FLAIR) (B) brain MRI on diagnosis (T1 with contrast) (C) 9-month follow up brain MRI (T1 with contrast) (D) GBM relapse, 12-month follow up brain MRI (T1 with contrast) (E) 30-month follow up brain MRI (T1 with contrast).
图 6 患者3: (A) 确诊时脑部MRI (T2/FLAIR) (B) 确诊时脑部MRI (增强T1) (C) 9个月随访脑部MRI (增强T1) (D) GBM复发, 12个月随访脑部MRI (增强T1) (E) 30个月随访脑部MRI (增强T1)。
4 Results
4 结果
Out of the 18 patients, 6 followed the diet for more than 6 months (Figure 10). The effect of diet on the disease evolution of these patients is shown in Table 4. More specifically, from the 6 patients who followed the diet for more than 6 months, one patient died at 43 months (thus achieving 3 years survival), one patient died exactly 36 months after diet initiation (thus narrowly missing 3 years survival), and one patient is still alive 33 months after the start of the diet (but has not yet reached the 3-year goal, and is not included in the final percentage). The remaining 3 are also still alive, completing 84, 43, and 44 months of life, respectively. Therefore, the 3-year survival rate .
在18名患者中,有6人坚持饮食疗法超过6个月 (图 10)。这些患者的饮食对疾病进展的影响如表 4所示。具体而言,在坚持饮食超过6个月的6名患者中:1名患者在43个月时去世(即实现3年生存),1名患者恰好在开始饮食疗法36个月后去世(以微弱差距未达3年生存目标),另1名患者在开始饮食33个月后仍存活(但尚未达到3年目标,故未计入最终百分比)。其余3名患者目前仍存活,分别完成84个月、43个月和44个月的生存期。因此,3年生存率为。
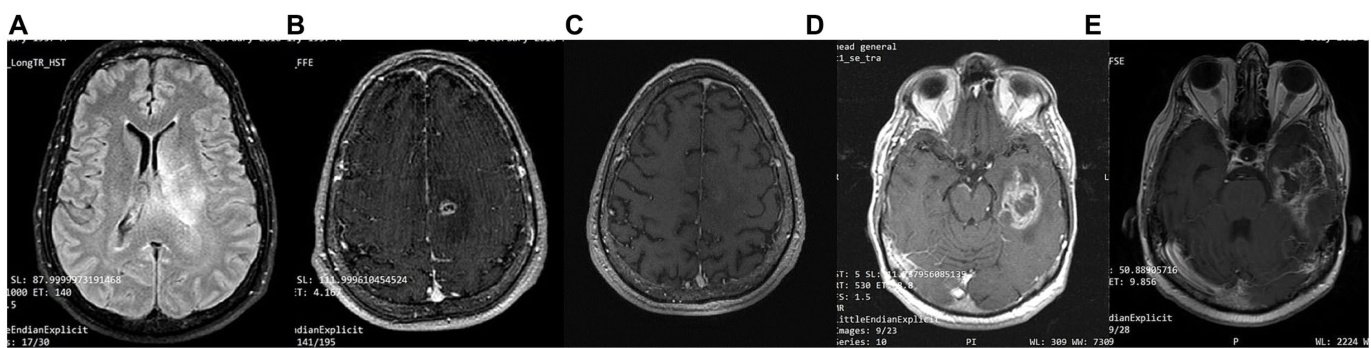
FIGURE 7 Patient 4: (A) brain MRI on diagnosis (T2/FLAIR) (B) brain MRI on diagnosis (T1 with contrast) (C) 24-month follow-up brain MRI (T1 with contrast) (D) 32-month follow up brain MRI. GBM relapse (T1 with contrast) (E) 41-month follow up brain MRI (T1 with contrast).
图 7: 患者4: (A) 确诊时脑部MRI (T2/FLAIR) (B) 确诊时脑部MRI (增强T1) (C) 24个月随访脑部MRI (增强T1) (D) 32个月随访脑部MRI。胶质母细胞瘤复发 (增强T1) (E) 41个月随访脑部MRI (增强T1)
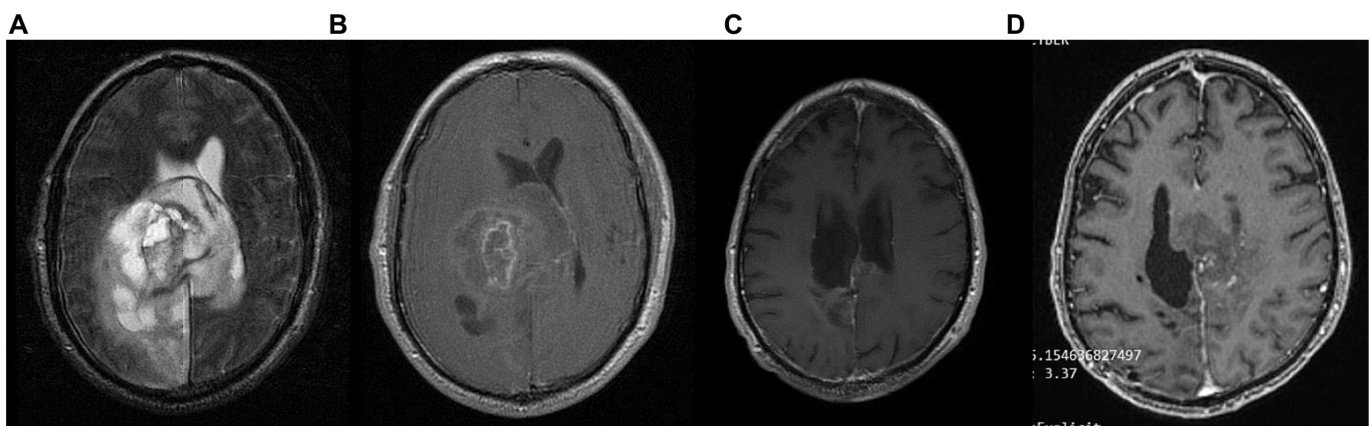
FIGURE 8 Patient 5: (A) pre-operative brain MRI (T2/FLAIR) (B) pre-operative brain MRI (T1 with contrast) (C) 34-month follow up brain MRI (T1 with contrast) (D) GBM relapse 40-month follow up brain MRI (T1 with contrast).
图 8 患者5: (A) 术前脑部MRI (T2/FLAIR) (B) 术前脑部MRI (T1增强) (C) 34个月随访脑部MRI (T1增强) (D) GBM复发40个月随访脑部MRI (T1增强)。
The characteristics of the patients who did not comply with the diet beyond 6 months are shown in Table 5. Of the 12 patients who did not adhere to the diet, only one reached 36 months of survival, while the rest have died in an average time of $15.7\pm6.7$ months, with a 3-year survival rate of $8.3%$ .
未坚持饮食超过6个月的患者特征如表5所示。在12名未遵守饮食方案的患者中,仅1例存活达到36个月,其余患者平均生存时间为$15.7\pm6.7$个月,3年生存率为$8.3%$。
Comparing the survival rates of the two groups, we see that the difference is $58.3%$ (66.7 versus $8.3%$ ) and is statistically significant with $p<0.05$ (0.0114) and $\mathrm{X}^{2}=6.409$ .
比较两组的存活率,我们发现差异为 $58.3%$ (66.7 对比 $8.3%$),且具有统计学显著性 ($p<0.05$) (0.0114) 以及 $\mathrm{X}^{2}=6.409$。
Unique features were observed in the 6 patients who adhered to the diet (Table 3). Patient 1 (P1) received a combination of radiation and $75:\mathrm{mg}/\mathrm{m}^{2}$ temozolomide for 1 month. He continued with maintenance temozolomide at $180:\mathrm{mg/m}^{2}$ , 5 days/month for 24 months and then discontinued it. In total, he received concurrent chemotherapy $+\mathrm{KD}$ for 24 months. Only KD was administered in the remaining observation time (60 months). His ECOG performance status was excellent with score 0 (fully active). Patient P2 is alive at 43 months, with 36 months under ketogenic diet therapy with ECOG 1.Patient P3 stopped the ketogenic diet and relapsed soon after. She was initially treated with chemotherapy and cortisone without an increase in her blood glucose levels, and after the end of chemotherapy, she resumed the ketogenic diet and since then recovered with ECOG 1. All 6 patients received chemotherapy and radiation, as well as corticosteroids, simultaneously with the ketogenic diet. It is remarkable that during this time, glucose values did not typically rise above $80\mathrm{mg/dL}$ . Comparatively, glucose values were particularly elevated at end-of-life care while on concomitant chemotherapy and corticosteroids in non-adhering patients.
在坚持饮食干预的6名患者中观察到独特特征(表 3)。患者1(P1)接受了放疗联合$75:\mathrm{mg}/\mathrm{m}^{2}$替莫唑胺治疗1个月,随后以$180:\mathrm{mg/m}^{2}$剂量维持治疗(每月5天),持续24个月后停药。总计接受同步放化疗$+\mathrm{KD}$治疗24个月,后续观察期(60个月)仅采用生酮饮食。其ECOG体能状态评分为0分(完全正常活动)。患者P2目前存活43个月,其中36个月接受生酮饮食治疗,ECOG评分为1分。患者P3中断生酮饮食后很快复发,初期接受化疗和可的松治疗时血糖未升高,化疗结束后重启生酮饮食,目前ECOG恢复至1分。6例患者均在接受生酮饮食期间同步接受放化疗及皮质类固醇治疗。值得注意的是,治疗期间血糖值通常未超过$80\mathrm{mg/dL}$。相比之下,未坚持饮食干预的患者在临终关怀期间接受联合化疗和皮质类固醇治疗时,血糖值显著升高。
5 Discussion
5 讨论
In our cohort, out of the 18 patients, 6 followed the diet for the predefined period of ${>}6$ months. The effects of the ketogenic diet observed in these six IDH-wild type GBM patients who adhered to the diet are promising, especially when compared to the disease progression in patients who were unable to maintain the diet, as well as to historical controls (1, 35–37). Two of them died after having lived 43 and 36 months, a period considerably longer than the average lifespan of patients with GBM (patient 4 and 6 respectively). The remaining 4 are still alive, with one completing 84 months of life (patient 1), while the other three remain alive at 43, 33 and 44 months (patients 2, 3, and 5 respectively). It should be noted that all adhering patients (with the exception of patient 1) continued intermittent chemotherapy. Therefore, the first question that arises is whether the ketogenic state contributed to the longer survival due to its effects on cancer metabolism, by potenti a ting chemotherapy, or both. We believe that the metabolic effects of the diet contributed to improved outcomes because the patients that did not receive it, or were not able to adhere to it, still receiving other standard of care treatments, exhibited shorter survival in our cohort.
在我们的队列中,18名患者中有6人坚持了预定义的 ${>}6$ 个月生酮饮食。这六名坚持饮食的IDH野生型胶质母细胞瘤(GBM)患者中观察到的效果令人鼓舞,尤其是与未能维持饮食的患者疾病进展以及历史对照数据相比 (1, 35–37)。其中两名患者分别存活43个月和36个月后离世(患者4和6),这一生存期显著长于GBM患者的平均生存期。其余4名患者目前仍存活,其中1人已生存84个月(患者1),另外三人分别存活43、33和44个月(患者2、3和5)。需注意的是,除患者1外,所有坚持饮食的患者均持续接受间歇性化疗。因此,首要问题是生酮状态通过影响癌症代谢、增强化疗效果,还是两者共同作用延长了生存期。我们认为饮食的代谢效应改善了预后,因为未接受或无法坚持该饮食但仍在接受其他标准治疗的患者,其生存期在